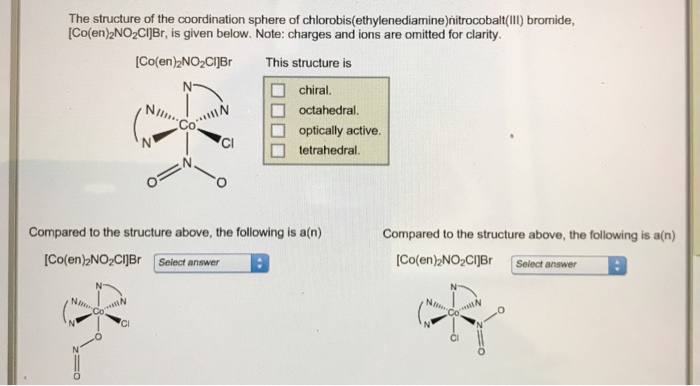
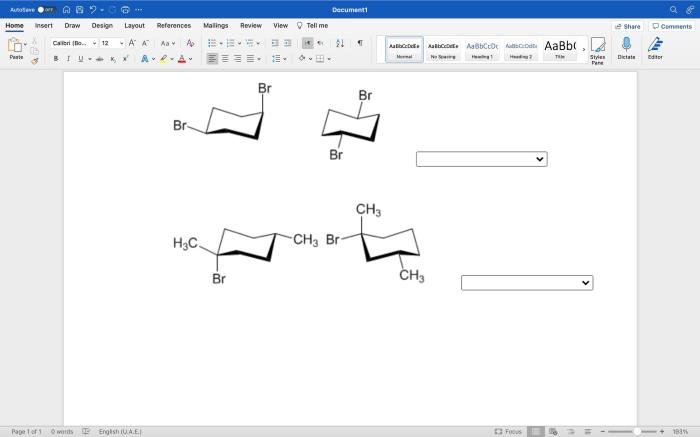
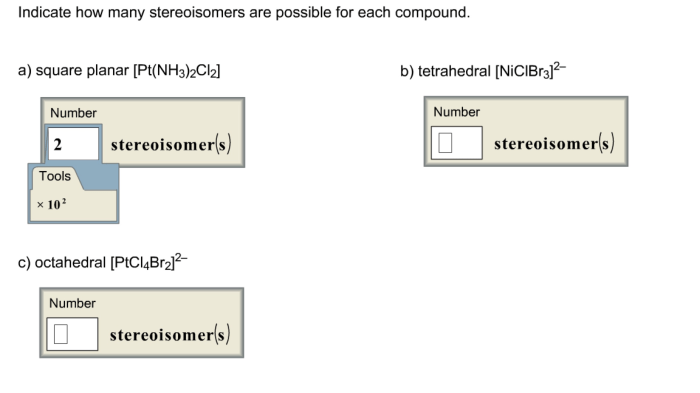
Indicate how many stereoisomers are possible for each compound. – In the realm of chemistry, stereoisomers emerge as molecules that share the same molecular formula and connectivity, yet differ in their spatial arrangement. Understanding the factors influencing the number of stereoisomers possible for a given compound unveils a fascinating chapter in the study of molecular structure and reactivity.
This discourse delves into the intricacies of stereoisomerism, exploring the impact of structural, geometric, and optical isomerism on the proliferation of stereoisomers. By unraveling the relationship between molecular architecture and the number of stereoisomers, we gain invaluable insights into the diversity and complexity of the chemical world.
Define Stereoisomers
Stereoisomers are molecules that have the same molecular formula and connectivity but differ in the spatial arrangement of their atoms.
Stereoisomers arise when there is restricted rotation around a double bond or when there is a chiral center in the molecule.
Structural Isomerism
Structural isomerism refers to compounds that have the same molecular formula but different structural formulas.
The number of stereoisomers for a structural isomer depends on the number of chiral centers in the molecule.
- A molecule with one chiral center can have a maximum of two stereoisomers.
- A molecule with two chiral centers can have a maximum of four stereoisomers.
Geometric Isomerism
Geometric isomerism refers to compounds that have the same molecular formula and connectivity but differ in the spatial arrangement of their atoms around a double bond.
The number of stereoisomers for a geometric isomer depends on the type of double bond in the molecule.
- A molecule with a carbon-carbon double bond can have a maximum of two stereoisomers (cis and trans).
- A molecule with a carbon-nitrogen double bond can have a maximum of three stereoisomers (E, Z, and N).
Optical Isomerism
Optical isomerism refers to compounds that have the same molecular formula and connectivity but differ in their ability to rotate plane-polarized light.
The number of stereoisomers for an optical isomer depends on the presence of a chiral center in the molecule.
- A molecule with one chiral center can have a maximum of two stereoisomers (enantiomers).
- A molecule with two chiral centers can have a maximum of four stereoisomers (diastereomers).
Combinations of Isomerism, Indicate how many stereoisomers are possible for each compound.
It is possible for a molecule to exhibit multiple types of isomerism.
The number of stereoisomers for a molecule with multiple types of isomerism is the product of the number of stereoisomers for each type of isomerism.
FAQ Section: Indicate How Many Stereoisomers Are Possible For Each Compound.
What is the relationship between the number of chiral centers and the number of stereoisomers?
The number of stereoisomers increases exponentially with the number of chiral centers. For a compound with n chiral centers, the maximum number of stereoisomers is 2^n.
How does geometric isomerism affect the number of stereoisomers?
Geometric isomerism occurs when a molecule has a double bond with restricted rotation. This restriction can lead to the formation of two or more stereoisomers, depending on the orientation of the groups attached to the double bond.
What is the difference between structural isomerism and stereoisomerism?
Structural isomers have the same molecular formula but different structural formulas. Stereoisomers have the same molecular formula and structural formula, but differ in their spatial arrangement.