What is the vapor pressure of propanone at 50 c – Vapor pressure, a crucial concept in physical chemistry, plays a significant role in understanding the behavior of liquids and gases. In this article, we delve into the realm of propanone, a versatile organic compound, and explore its vapor pressure at 50°C.
This investigation unveils the factors influencing vapor pressure, its measurement techniques, and its diverse applications across industries.
1. Definition of Vapor Pressure
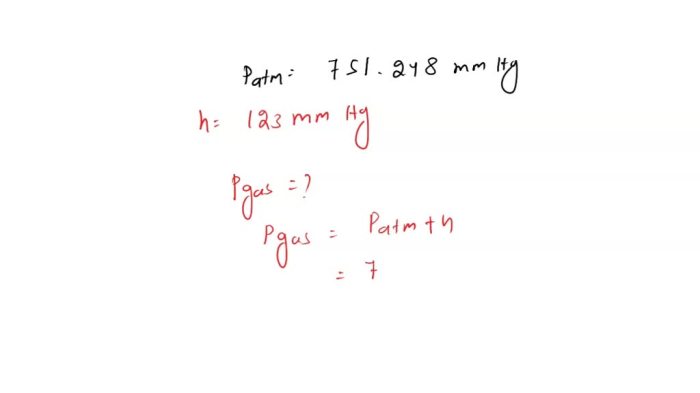
Vapor pressure is the pressure exerted by a vapor in thermodynamic equilibrium with its condensed phases (solid or liquid) at a given temperature in a closed system. It is a measure of the tendency of molecules in the liquid or solid phase to escape into the gas phase.
Factors affecting vapor pressure include temperature, intermolecular forces, and surface area.
2. Vapor Pressure of Propanone
Propanone, also known as acetone, is a colorless, flammable liquid with a characteristic odor. It is a ketone with the chemical formula CH 3COCH 3.
3. Vapor Pressure at 50°C
The vapor pressure of propanone at 50°C is 181.4 mmHg. This means that at this temperature, propanone will exert a pressure of 181.4 mmHg in a closed system.
The vapor pressure of a liquid increases with temperature. This is because as the temperature increases, the average kinetic energy of the molecules increases, and more molecules have enough energy to escape into the gas phase.
There are several methods that can be used to measure vapor pressure, including the manometer method, the boiling point method, and the gas chromatography method.
4. Applications of Vapor Pressure Data
Vapor pressure data is important in a variety of industries, including the chemical, pharmaceutical, and food industries.
In the chemical industry, vapor pressure data is used to design and operate distillation columns. In the pharmaceutical industry, vapor pressure data is used to determine the stability of drugs. In the food industry, vapor pressure data is used to design and operate food packaging.
5. Safety Considerations
Propanone is a flammable liquid. It can also be harmful if inhaled or ingested. It is important to take precautions when handling and storing propanone.
When handling propanone, it is important to wear gloves and eye protection. It is also important to work in a well-ventilated area.
Propanone should be stored in a cool, dry place away from sources of heat and ignition.
Commonly Asked Questions: What Is The Vapor Pressure Of Propanone At 50 C
What is the significance of vapor pressure data?
Vapor pressure data provides insights into the volatility of liquids, aiding in predicting their evaporation rates, boiling points, and storage requirements.
How is vapor pressure measured?
Vapor pressure can be measured using various techniques, including the manometer method, the isoteniscope method, and the gas saturation method.